Automation Solutions
As FactoryTR, we offer process automation solutions that comply with GMP and 21 CFR Part 11 requirements.
- We design and install state-of-the-art automation systems to ensure that production processes are traceable, safe, repeatable and efficient, especially in the pharmaceutical, food and cosmetics sectors.
- In today's industry, keeping hygienic processes under full control, data security and traceability are of great importance.
- Systems that comply with the requirements of regulatory bodies such as GMP and FDA minimize the risk of errors while increasing production quality.
- Specifically, the 21 CFR Part 11 requirement dictates that electronic records and signatures must be reliable, immutable, and auditable.
- As FactoryTR, we design and implement all our automation solutions in accordance with these standards.
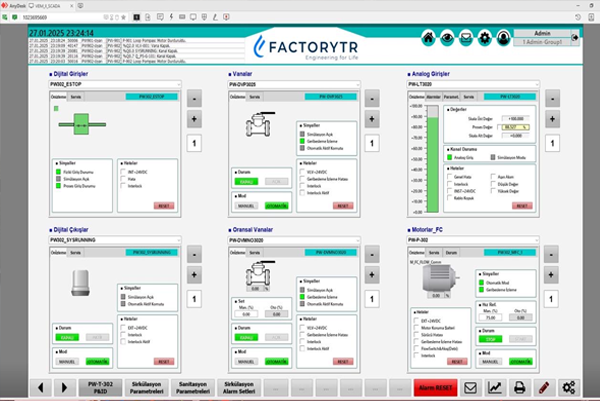
Automatic Production and Traceability Systems
- Prescription management and lot tracking
- Real-time monitoring and recording of production processes
- Control of sealing, temperature, pressure, flow and other critical process parameters
GMP and 21 CFR Part 11 Compatible SCADA and PLC Systems
- Central monitoring and management of production processes with SCADA systems
- Optimizing process control with PLC solutions
- Electronic data recording and secure archiving in accordance with 21 CFR Part 11 requirements
- Authorized user access management and electronic signature applications
Process Safety and Data Management
- GMP compliant alarm and error monitoring systems
- Data integrity, immutability and access control in accordance with 21 CFR Part 11
- Full traceability with audit trail records
Validation and Documentation Services
- IQ (Installation Qualification), OQ (Operational Qualification), PQ (Performance Qualification) tests
- Validation processes in accordance with GAMP 5 (Good Automated Manufacturing Practice) requirements
